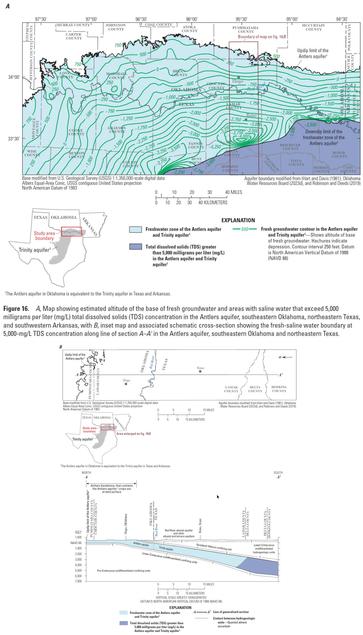
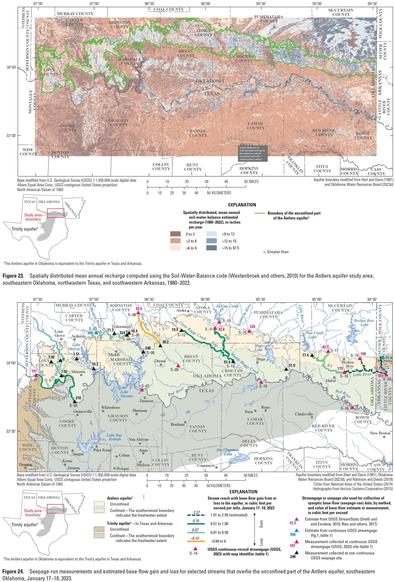
Hydrogeologic Investigation, Framework, And Conceptual Flow Model Of The Antlers Aquifer, Southeastern Oklahoma, 1980–2022
--
https://doi.org/10.3133/sir20255013 <-- shared USGS publication
--
https://waterdata.usgs.gov/nwis <-- shared USGS National Water Information System (NWIS) database
--
https://doi.org/10.5066/P14C6QFS <-- shared associated USGS open data release
--
#GIS #spatial #mapping #fedscience #water #hydrology #wateresources #pump #pumping #groundwater #AntlersAquifer #aquifer #Oklahoma #model #flow #test #flowmodel #yield #wateruse #watersecurity #climate #weather #recharge #precipitation #rainfall #TDS #waterquality #ions #potentiometric #geology #monitoring #planning #regulatory #agriculture #farming #cropland #spatialanalysis #spatiotemporal #fedscience
@USGS
Recent searches
Search options
#ions
In 2023, #SpaceX launched the first of its #Starlink V2 Mini satellites
. These next-generation spacecraft are equipped with #HallEffect thrusters, or #HETs, that operate on #argon, instead of the #krypton propellant on previous Starlinks https://aerospaceamerica.aiaa.org/year-in-review/a-year-of-firsts-for-electric-propulsion
The essential working principle of the #HallThruster is that it uses an #electrostatic potential to accelerate #ions up to high speeds https://en.wikipedia.org/wiki/Hall-effect_thruster#Principle_of_operation
https://www.europesays.com/1761489/ New technique uses hydrogen to tune exotic materials for quantum devices #CityCollegeOfNewYork #Data #ElectricCharge #hydrogen #Ions #QuantumAnomalousHall #QuantumDevices #WeylFermions #WeylSemimetals
Australia's Massive Wildfires Shredded the Ozone Layer--Now Scientists Know Why
Massive wildfires that raged across southeast Australia in 2019–20 unleashed chemicals that chewed through the ozone layer, expanding and prolonging the ozone hole.
A study, published today in Nature, describes how smoke combined with chlorine-containing molecules in the stratosphere — remnants of chemicals that are now banned — to cause the destruction.
The Australian fires produced the largest smoke plume on record, releasing roughly one million tonnes of smoke to heights of up to 30 kilometers.
That’s well into the stratosphere, the portion of the atmosphere that contains the ozone layer, which protects Earth from harmful ultraviolet rays, says study co-author Kane Stone, an atmospheric chemist at the Massachusetts Institute of Technology (MIT) in Cambridge.
In the months after the wildfires, the hole in the ozone layer, which appears annually over Antarctica, was larger and lasted longer than in previous years.
About 80% of the chlorine in the atmosphere is a legacy of #chlorofluorocarbons, chemicals used in aerosol sprays and as refrigerants starting in the 1930s.
Their use has mostly been phased out since an international treaty was implemented in 1987. Remnant chlorine is bound up as #hydrochloric #acid and #chlorine #nitrate, which are harmless to the ozone layer.
But when #hydrochloric #acid dissolves in #water droplets, it forms reactive ozone-depleting molecules.
That doesn’t usually happen away from the poles, because the air is too #warm, says Stone.
The team used a computer model to predict how various organic acids contained in smoke particles would alter the solubility of hydrochloric acid.
The changes produced in the simulations mirrored the changes to stratospheric chemistry that were observed after the fires.
Solomon says that #hydrochloric #acid latches onto the #surface of the #smoke #particles and reacts with other molecules to produce #molecular #chlorine, which is broken down in sunlight to highly reactive ‘ozone-eating’ chlorine #ions.